By April Day, Publishing Director for Save The Water™ | September 9, 2018
Electroflocculation: What is it? A Way to Treat Water with Few Chemicals.
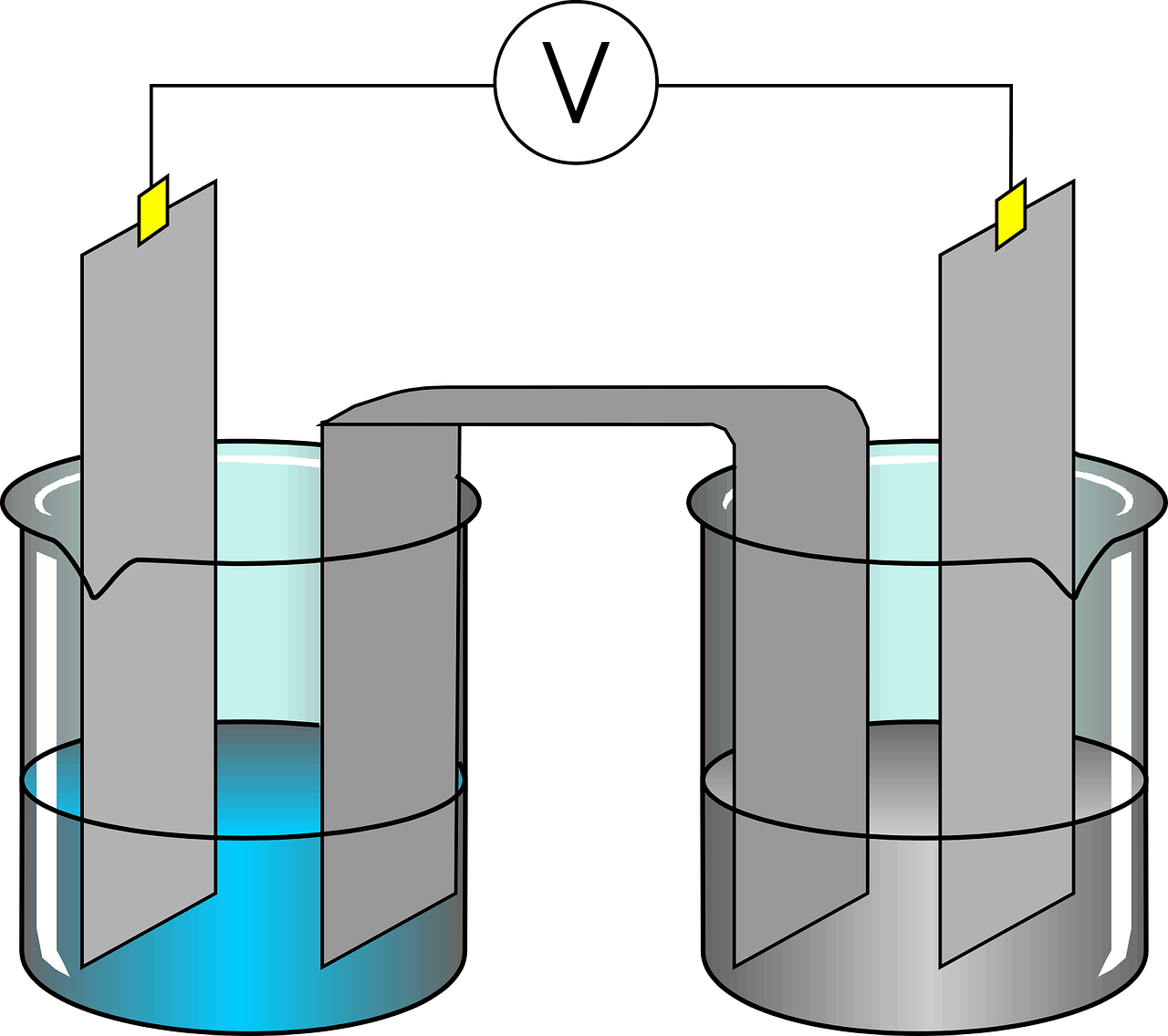
Electroflocculation is a process that uses electricity to remove contaminants from wastewater.1 The name has two parts, electricity and flocculation. The “flocculation” part means the process of creating “flocs.” “Flocs” are masses made by an aggregation of fine particles.2 Now, let’s turn to the electricity part. First, we need to understand the basics of an electric cell. For an electric cell in electroflocculation, we need at least three things: wastewater and two electrodes, namely, a cathode and an anode. Unlike the conventional electric cell, in electroflocculation, the cathode is inert, meaning that it does not react with the solution.
Usually, electrodes are pieces of metal, namely, a cathode which changes from a positively charged metal ion into a non-charged metal and an anode.3 In electroflocculation, however, for the cathode, two water molecules (H2O) and two negatively charged electrons, “reduce” into two negatively charged hydroxide molecules (2OH-) and a hydrogen (H2) molecule. The other metal, the anode, say aluminum (Al), changes from a metal with electrons (Al-3e) to a metal ion with a positive charge (Al3+). The process at the anode is known as “oxidizing.” When the anode, here, the aluminum, oxidizes, it releases electrons. The metal that oxidizes also disappears.4 The electrons released from the anode do not have a place to attach so these electrons attract the contaminants in the wastewater to themselves, forming bubbles at the cathode.5
These charged ions attract pollutants without changing the water’s pH. These pollutants “flocculate,” forming larger masses that separate from the water, either gathering at the bottom or floating to the top in gas bubbles.6,7 The pollutants are then easily removable from the water.6
This process uses the anode until it does not do its job anymore, so it must be replaced.
We Need a Safe, Effective, and Cheap Way to Treat Water So We Can Use It.
Wastewater or untreated water can contain different contaminants that make it unsafe for people to use, such as:
- Clay
- Heavy metals
- Asbestos
- Starch
- Organic molecules with a molecular weight greater than ≈ 300 gram-per-mole
- Free and emulsified fats, oils, greases (also known as “FOG”)
- Hydrocarbons, including benzene ring structures and many halogenated hydrocarbons
- “Sticky” substances, such as glues, polymers, and monomers
- Cations with Z > 23 and some lower Z, such as Mg and Ca
- Anions with Z > 33 and some with lower Z, such as F
- Chemicals such as cyanide, arsenate, and phosphate
- Pathogens such as algae, bacteria, and viruses
- BOD/COD
- Many dye molecules, tannins, and humic substances
- Soaps and detergents6
- Endocrine disrupting chemicals
Many of these contaminants, in certain amounts, can cause diseases that hurt people and may lead to death.
Electroflocculation Uses Recognized Technology in a Way that Uses Lesser Amounts of Chemicals and Has Lower Operating Costs.
Using traditional technology, contaminants are removed from water by adding chemicals to the water. First, this use of chemicals can leave additional chemicals, such as chlorine, in the water and can change the water’s pH, making it unusable for certain applications, such as agriculture. Second, adding chemicals to the water also requires a higher level of expertise that has been acquired by highly trained personnel to run the process. Third, this process requires a continuous use of chemicals, resulting in higher costs for materials.8
Alternatively, reverse osmosis (RO) presents another way to remove contaminants and pollutants from water. You can read about reverse osmosis and desalination here. Although reverse osmosis is good at removing contaminants, it costs a lot of money in energy costs. Also, reverse osmosis is unable to handle highly contaminated water with clays, oil and grease, and suspended solids. That said, reverse osmosis is very good at treating tap water to remove the final contaminants after conventional treatment. Reverse osmosis depends on membranes that are often made from expensive materials. Larger pollutants collect on the membrane, making it dirty and unusable. Therefore, the membrane is either ruined and must be cleaned or replaced, also costing a lot of money.8
Electroflocculation does not have these problems. Depending on the cathode and anode materials used, the materials are not expensive. Materials that can be used for the sacrificial anode include magnesium, iron, and aluminum.9 After a time, the sacrificial anode must be replaced. But the process requires less operational costs in terms of personnel and replacing materials compared to other processes. Also, electroflocculation can be used as a pretreatment for water before reverse osmosis. Moreover, using direct current electricity instead of alternating current electricity can contribute to cost reduction.10
Action: Using Electroflocculation is a Solution to Many Water Treatment Challenges.
Electroflocculation presents a way to treat water in an effective, relatively safe, and economically feasible manner. The proprietary technologies that Save the Water™ offers can be licensed. The water produced by Save the WaterTM’s technologies meet the U.S. Drinking Water standards. If you want to know more about licensing, contact us here,or donate to Save the WaterTM here.
References
- Vivian Robinson. January 1999. “Electroflocculation in the Treatment of Polluted Water.” https://bit.ly/2QbjMrs
- Merriam-Webster Dictionary (online). Definition of floc. https://www.merriam-webster.com/dictionary/floc
chemisNATE. January 31, 2013. “What’s the Anode, Cathode, and Salt Bridge?”
https://www.youtube.com/watch?v=a2v7ph3kLXo - Suez UK. January 25, 2016. “Wastewater Treatment: Electrocoagulation, Electrooxidation, Electroperoxi-coagulation – SUEZ.” https://www.youtube.com/watch?v=w6G09RjD5zM
- Eslam Abdullah. December 31, 2013. “IET Industrial Effluent Treatment Electroflocculation.” https://www.youtube.com/watch?v=_kFQ-6X51Lk
- Dr. Vivian Robinson. “Electroflocculation: Wastewater Treatment by Electroflocculation.” Published in Chemistry and Material Science Encyclopaedia of Applied Electrochemistry, on Soneera Water, https://bit.ly/2PGCwy4
- Miu mew. April 9, 2014. “Our Water Story: Electrocoagulation.” MWIT Studio, Producers Natnicha Plongmai and Tanya Kurutach. https://www.youtube.com/watch?v=qc-DtdzYeiE
- David H. Paul, Inc. August 16, 2017. “Electrocoagulation as Pretreatment for Reverse Osmosis.” https://www.youtube.com/watch?v=WO8qtkLcmZo
- Franziska Bleeke, et al. August 21, 2015. “Effect of voltage and electrode material on electroflocculation of Scenedesmus acuminatus.”Bioresources and Bioprocessing. https://bit.ly/2oKYuVf
- A.A. Cerqueira, et al. 2014. “Effects of direct and alternating current on the treatment of oily water in an electroflocculation process.” Brazilian Journal of Chemical Engineering, 31(3). http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0104-66322014000300012