By Leigh Horton, staff writer for Save The Water™ | May 31, 2014
Introduction
This report synthesizes information about the effects of Methyl tertiary butyl ether (MTBE) on ground water and public drinking wells. It is based solely on a literature review of relevant materials from predominantly American organizations researching wells in the United States. This report is a data-based investigation of MTBE contamination.
Background
In the late 1970’s, MTBE was occasionally used as a gasoline additive to improve engine efficiency by increasing octane levels. The Clean Air Act Amendments introduced in 1990 to reduce smog and other pollutants resulted in an increased use of MTBE.3 These amendments did not require that oil companies add MTBE specifically, but economic consideration led most of them to add MTBE to their gasoline instead of alternative oxygenates. After these regulations were passed, groundwater readings began to reveal that MTBE exceeded the maximum contamination levels. In regions where MTBE contamination was the most noticeable (like California and Colorado, among others), residents began complaining about undrinkable water supplied by cities and private wells. Investigations were conducted by private and federal organizations. Their findings indicated that the additional MTBE usage was most likely contributing to water contamination. Their results contributed to the reduction of MTBE use in gasoline. In 2005, the US government no longer required reformulated gasoline to have oxygenates like MTBE, and state bans substantially reduced the amount of MTBE used.
Definitions
- Aerobic biodegradation: “the breakdown of organic contaminants by microorganisms when oxygen is present”.4
- Methyl tertiary butyl ether (MTBE): A gasoline additive used to improve gasoline octane levels.
- Clean Air Act Amendments: Amendments to the Clean Air Act originally passed in 1970 and amended in 1990. It required that oxygenates be added to gasoline to improve engine efficiency and thereby reduce air pollution.
Methods
This report is based on a literature review of sources from the Environmental Protection Agency and other government agencies and hearings. The sources referenced in this report are listed in the citations section. For the sake of brevity, not all the literature read is included in this report, only the sources referenced.
Findings
Major Sources and the Extent of MTBE in Ground Water
Benjamin H. Grumbles, the Deputy Assistant Administrator for Water for the EPA in 2002, stated that “the most significant sources of contamination of water resources [from MTBE] are from leaking underground and above ground storage tanks, pipelines, refueling spills, emissions from older marine engines, and to a much lesser degree, storm runoff and precipitation”.5 The USGS conducted research that concluded that high concentrations of MTBE contamination come from leaking underground storage tanks, common structures used at gas stations or any other facility containing hazardous materials.5 The USGS also found that in their 31-state study, 9,000 wells may be within a 1 kilometer radius of a leaking underground storage tank.5 However, not all wells near the tanks are at risk of contamination. Contamination is determined by the direction of groundwater flow, geologic properties, and other factors.
Reported Health Problems
The most common complaint has been the taste-and-smell-quality of drinking water. The health effects are minimal but include respiratory issues near MTBE sources, headaches, coughing, burning throat or nose, eye irritation, nausea or vomiting, disorientation, and dizziness.1
MTBE Behavior in Ground Water
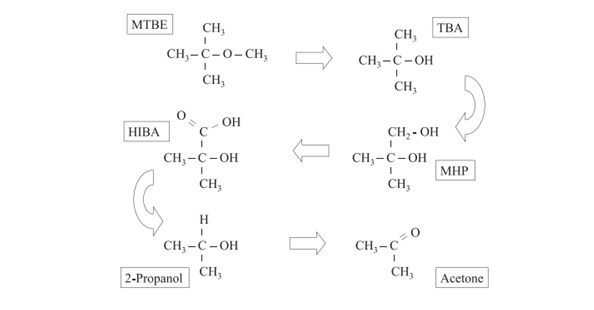
MTBE is highly soluble in water and in certain environments, it can biodegrade “in ground water and soil where sufficient oxygen is present and in bed sediments of streams, lakes, wetlands, and estuaries”.5 However, this aerobic biodegradation turns MTBE to acetone (see Figure 1).
Figure 1: “Significant products of the aerobic biodegradation of MTBE. (After Wilson 2003b). MHP is methyl-2-hydroxy-1-propanol. HIBA is 2-hydroxyisobutyric acid”.6
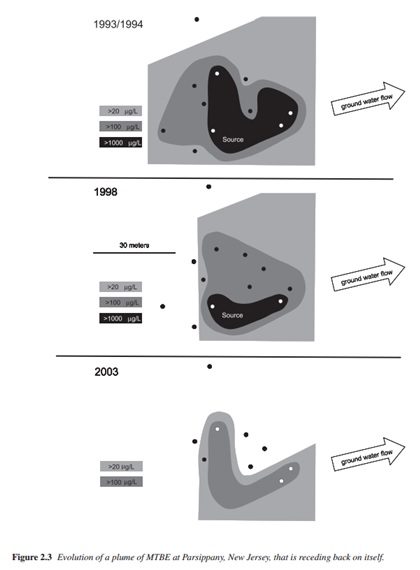
MTBE also moves readily with ground water (see Figure 2). The EPA states that the length of the plume correlates to MTBE concentration. The higher the concentration, the larger the plume will be and vice versa. However, the plume length is difficult to predict after a spill because of the variability of MTBE concentration in gasoline.6 Initial concentrations of MTBE in the gasoline are not uniform. In other words, some gasoline may contain 11% MTBE (a standard value) but another batch of gasoline may only contain 9% MTBE. Therefore, the plume from the gasoline containing 11% MTBE will be larger than that formed by the 9% MTBE gasoline. Additional factors are characteristics of the aquifer through which the MTBE flows. The EPA states that “concentration of MTBE in a well can change through the combined influence of dilution and dispersion, biodegradation, sorption, and mixing of the contaminant plume with cleaner water in a monitoring well.” Their research also indicates that the addition of MTBE does not change the ground water movement.6 Finally, the EPA indicates that “the size of a plume reflects a balance between the rate of release of the contaminant from the source area into the aquifer, the rate of transport of the contaminant away from the source area, and the rates of degradation and dispersion in ground water which remove mass or reduce plume concentration”.6
Discussion
Since ground water can readily transport MTBE, many private and public wells have been increasingly contaminated since the Clean Air Act Amendments in 1990. Since 2005, the amount of MTBE added to gasoline has dramatically reduced because of state bans and federal regulations. However, the past sources for MTBE are still problematic. Leaking underground and above ground storage tanks should be regulated, especially those that still store gasoline enhanced or unenhanced with MTBE. Gasoline spills should be treated with care and studied to further determine how gasoline moves through aquifers. The health problems associated with exposure to MTBE are not life-threatening in most cases. Physical irritation and taste and smell discomfort from drinking water are generally the extents of the health problems.1 Since MTBE becomes acetone in ground water after biodegradation, there is a concern of cellular damage and unconsciousness from the increased amount of acetone.2These effects were impermanent. Hospitalization and time were able to cure the effects of acetone exposure.1
Conclusion
MTBE is no longer commonly used as a gasoline oxygenate. In 2005, reformulated gasoline was not required to contain oxygenates and state and federal governments dramatically limited MTBE use. However, more studies are needed to determine if MTBE is still present in ground water used for public consumption. Leaking tanks should be addressed and gasoline spills studied with care. Despite the fact that MTBE does not pose a life-threatening risk, it is still a contaminant that should be eradicated quickly for the public good. Furthermore, alternatives to MTBE like ethanol should be explored and used when an oxygenate is needed for octane improvement.
References
- Agency for Toxic Substances and Disease Registry. (May, 2014). “Health Effects.” Retrieved from http://www.atsdr.cdc.gov/ToxProfiles/tp91-c2.pdf
- New Energy and Fuel. (October, 2012). Evonik Plant Photo. Image. Retrieved from http://newenergyandfuel.com/http:/newenergyandfuel/com/2012/10/05/a-bio-sourced-form-of-mtbe-is-introduced/
- United States Environmental Protection Agency. (May, 2013). “Methyl Tertiary Butyl Ether (MTBE).” http://www.epa.gov/mtbe/faq.htm
- 3United States Geologic Survey. (February, 2013). “Aerobic Biodegradation.” Retrieved from http://toxics.usgs.gov/definitions/aerobic_biodegradation.html
- U.S. Government Printing Office. (May, 2002). “MTBE Contamination in Groundwater: Identifying and Addressing the Problem.” Retrieved from http://www.gpo.gov/fdsys/pkg/CHRG-107hhrg80670/pdf/CHRG-107hhrg80670.pdf
- Wilson, John T., Philip M. Kaiser, and Cherri Adair. (January, 2005). “Monitored Natural Attenuation of MTBE as a Risk Management Option at Leaking Underground Storage Tank Sites.” United States Environmental Protection Agency. Retrieved from http://www.epa.gov/nrmrl/pubs/600r04179.html