By April Day, Publishing Director for Save The Water™ | February 16, 2020
What Do I Need to Know About PFAS?
Let’s go over some key facts you need to know about PFAS and PFAS treatments. To begin with, PFAS stands for “per and polyfluoroalkyl substances.” Generally, people use PFAS in many things such as fire-fighting activities, stain-repellent, and non-stick cookware. For example, in the United States, the Environmental Protection Agency has approved over 6,000 PFAS compounds for use and sale.
PFAS have carbon-fluorine (C-F) bonds, which are the shortest and strongest in chemistry. Because of its chemical makeup, PFAS remain stable. Second, PFAS don’t break down in nature. Third, PFAS stay around a long time.
Most importantly, PFAS build in living creatures, such as humans. According to research, PFAS can increase cholesterol levels in humans. Also, PFAS have been linked to cancer.
Vocabulary: What Words Do I Need to Know to Understand PFAS Treatments?
- Adsorption is a process that chemically (or physically) binds a molecule (liquid form) to a surface that is solid. People can use this process to get rid of a chemical in a liquid such as water.
- Activated carbon is carbon that is made up of small, black beads or a solid black sponge. The carbon is treated with oxygen. This makes the carbon really easy to pass through (porous).
- GAC stands for “Granular Activated Carbons.” Specifically, it is a water treatment that uses adsorption onto activated carbon media.
- AIX stands for “Anion Exchange Resin.” First, an anion is a negatively charged ion (molecule or atom with a negative charge). Second, resin is made up of organic polymers (substance made from a certain process with repeating chemical structures). In AIX, negative ions are attracted to a resin that is positively charged.
- Long-chain PFAS have 6 or more Carbon-Fluorine (C-F) bonds.
- Short-chain PFAS have 7 or less C-F bonds for certain types called carboxylates, and 5 or less for other types called perfluoroalkyl sulfonates.
How Can We Remove PFAS From Drinking Water With PFAS Treatments?
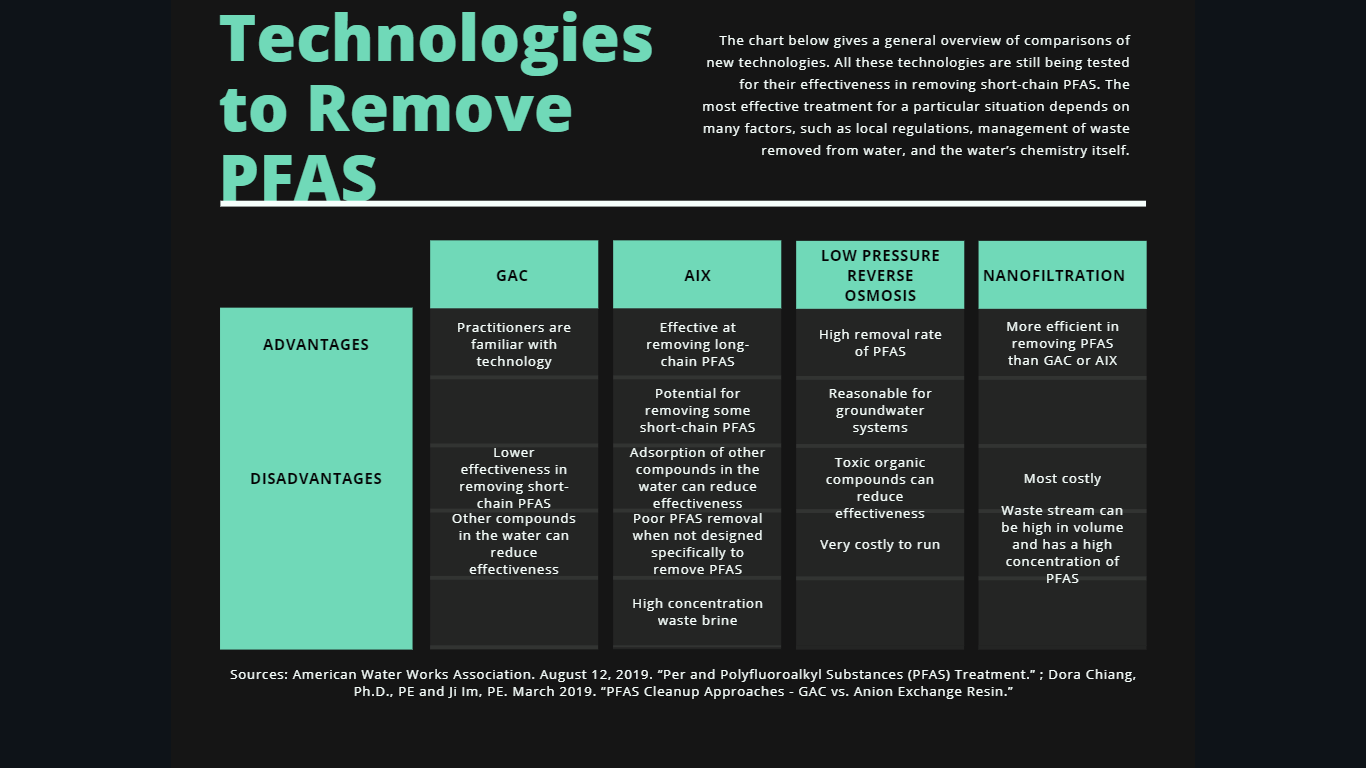
In short, conventional water treatment technologies don’t fully remove or destroy PFAS. However, many new technologies can. The chart below gives a general overview of the comparisons. Scientists continue to test all these technologies for their effectiveness in removing short-chain PFAS. To be sure, the most effective treatment for a particular situation depends on many factors, such as local regulations, management of waste removed from water, and the water’s chemistry itself.
What You Can Do
In addition, researchers continue to look at other technologies for treating PFAS, including electrocoagulation, which is a process that treats water by adding metal ions from sacrificial anodes (negative electrode where an electrical current enters a charged device) to remove pollutants. You can find out more about electrocoagulation, also known as electroflocculation, here. Stay updated by following Save The WaterTM on Twitter!
References
-
- American Water Works Association. August 12, 2019. “Per and Polyfluoroalkyl Substances (PFAS) Treatment.”
- Dora Chiang, Ph.D., PE and Ji Im, PE. March 2019. “PFAS Cleanup Approaches – GAC vs. Anion Exchange Resin.”
- Cheryl Hogue. August 24, 2019. “Short-chain and long-chain PFAS show similar toxicity, US National Toxicology Program says.” c&en Chemical & Engineering News. https://cen.acs.org/environment/persistent-pollutants/Short-chain-long-chain-PFAS/97/i33
- Anne Marie Helmenstine, PhD. July 11, 2019. “Activated Charcoal and How it Works.” ThoughtCo. https://www.thoughtco.com/how-does-activated-charcoal-work-604294
- chemistNate. January 13, 2013. What’s the Anode, Cathode, and Salt Bridge? YouTube. https://www.youtube.com/watch?v=a2v7ph3kLXo
- Vivian Robinson. January 1999. Electroflocculation in the Treatment of Polluted Water. Reseachgate.net. https://www.researchgate.net/publication/265990355_Electroflocculation_in_the_Treatment_of_Polluted_Water
- Y. Artoli. 2008. “Adsorption.” Encyclopedia of Ecology. https://www.sciencedirect.com/topics/earth-and-planetary-sciences/adsorption
Merriam-Webster Dictionary. Polymer. https://www.merriam-webster.com/dictionary/polymer
- SAMCO. November 28, 2017. “What Is Ion Exchange Resin and How Does It Work?” https://www.samcotech.com/ion-exchange-resin-work-process/




